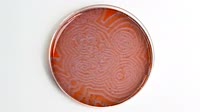
Timelapse footage of waves of colour produced in an oscillating BZ chemical reaction. The colours here are due to the indicator chemical ferroin, which contains an iron ion surrounded by three phenanthroline ligands. Ferroin is red when the iron is iron (II), in its +2 oxidation state, and blue when it is oxidised to the +3 state, iron (III). The initial mixture contains ferroin with iron (II), bromate ions, and malonic acid. Through a series of complex reactions, bromate oxidises the iron (II) to iron (III), turning it blue. The malonic acid reduces the iron (III) back to iron (II), turning it red again. Hypobromous acid in the solution reacts with malonic acid to produce bromomalonic acid and bromate ions. The varying concentrations of these species lead to their diffusion into regions of relative scarcity, and this, combined with the varying equilibrium positions of the reaction in adjacent areas, produces the appearance of travelling waves. The reaction was discovered independently by Boris Belousov in the 1950s and Anatoly Zhabotinsky in 1961, and is named in their honour.
Details
WebID:
C01839804
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:59.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No











 Loading
Loading