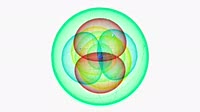
Zinc atom. Diagram of an atom of the element zinc, pulling back from the central nucleus to reveal the surrounding electron orbitals. Zinc is the 30th element, and has 30 protons (red) in its nucleus, along with most commonly 34 neutrons (blue), although it has many isotopes. Zinc has 30 electrons, and each of its orbitals can hold a maximum of two. Orbitals are filled from the lowest energy (nearest the nucleus) first, so there are two electrons in the inner 1s orbital, and two in the higher energy 2s orbital (green spheres), and two each in the slightly higher energy 2p orbitals (blue, red and yellow). Outside the 2p orbitals is a spherical 3s shell, and three 3p shells (magenta, blue and brown). At a higher energy is a spherical 4s orbital, and then five 3d orbitals. The 3d orbitals are arranged as three X-shaped orbitals arranged in the x, y and z plane (red, green and yellow), one X-shaped orbital in the x plane but at 45 degrees to the other (blue), and one two-lobed orbital in the z plane, with a small ring around the nucleus (purple). For clarity, solid lines show the paths of the electrons. In reality, electrons can be found anywhere, and the shape of each orbital is based only on where it is most likely to be found.
Details
WebID:
C01840012
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:40.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No










 Loading
Loading