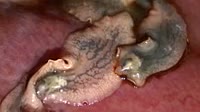
Belousov-Zhabotinsky (BZ) reaction. Footage demonstrating a Belousov-Zhabotinsky (BZ) reaction, involving bromine and methylmalonic acid. A solution containing bromate ions and methylmalonic acid is mixed in a beaker using a magnetic spinner, with cerium (IV) ions as a catalyst. The reaction mixture oscillates in colour between red-brown and pale blue in two auto-catalytic processes. Bromate ions are reduced to molecular bromine (red-brown), and then to bromide ions in the presence of cerium ions. The ratio of concentration of cerium (III) and cerium (IV) ions oscillates. This is an example of a class of reactions called Belousov-Zhabotinsky (BZ) reactions and is an example of non-equilibrium thermodynamics, and a non-linear chemical oscillator.
Details
WebID:
C01841646
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:01:00.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No












 Loading
Loading