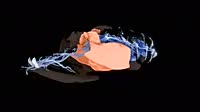
Solubility of methanol, the lightest alcohol. Alcohols are categorised by their functional -OH (hydroxyl) group. This functional group is highly polar and allows the alcohol to form strong intermolecular forces with other polar molecules, such as water. Here, methanol (CH3OH) is added to distilled water. Due to its low molecular size and highly polar hydroxyl group, the methanol molecules readily form strong intermolecular hydrogen bonds with polar water molecules. This produces one immiscible layer as the two liquids are mixed. See clip K004 1197 for the same process with a heavier alcohol, hexanol.
Details
WebID:
C01808757
Clip Type:
RM
Super High Res Size:
1920X1080
Duration:
00:00:17.000
Format:
QuickTime
Bit Rate:
25 fps
Available:
download
Comp:
200X112 (0.00 M)
Model Release:
NO
Property Release
No












 Loading
Loading